
Home » Prescient Surgical Achieves FDA De Novo Clearance for CleanCision System
Prescient Surgical Achieves FDA De Novo Clearance for CleanCision System
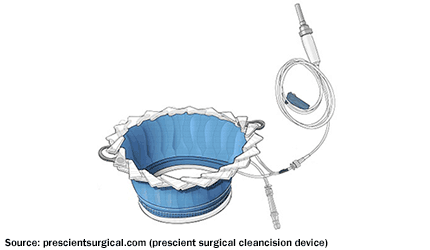
FDA’s CDRH has granted de novo clearance to Prescient Surgical’s CleanCision wound retraction and protection system.
The device is designed for surgical wound edge protection, retraction, and continuous cleansing with a sterile irrigant solution.
Prescient plans to launch the CleanCision device in 2017. — Cynthia Jessup
Upcoming Events
-
07May
-
14May
-
30May

