
Home » Neural Analytics Receives CE Mark for the Lucid System
Neural Analytics Receives CE Mark for the Lucid System
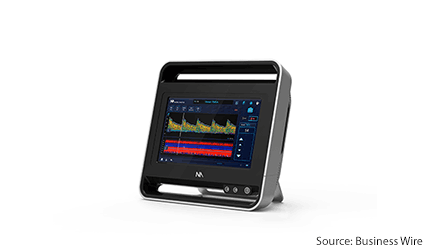
Los Angeles–based, Neural Analytics has gained a CE Mark for its advanced ultrasound device, the Lucid M1 transcranial doppler ultrasound system (lucid system), which is a portable all-in-one ultrasound system designed for rapid triaging and monitoring of patients with brain disorders.
The device system is a battery-operated, medical-grade tablet. It uses a type of ultrasound called transcranial doppler to assess the brain’s blood vessels from outside the body.
The device received FDA marketing clearance in October 2016. — Cynthia Jessup
Upcoming Events
-
07May
-
14May
-
30May

