
Home » Scopis Wins FDA Clearance and Health Canada MDL Clearance for Its Surgical Navigation System
Scopis Wins FDA Clearance and Health Canada MDL Clearance for Its Surgical Navigation System
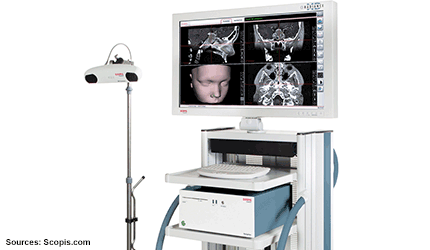
Massachusetts–based Scopis has received FDA marketing clearance and a Health Canada Medical Device License (MDL) for its surgical navigation products for ear, nose and throat surgery.
The technology is based on a small and portable unit that supports optical, electromagnetic and simultaneous hybrid tracking.
The modular device can be adapted for various uses. — Cynthia Jessup
Upcoming Events
-
25Apr
-
07May
-
14May
-
30May

