Claret Medical’s Sentinel Cerebral Protection System Wins CE Mark
Claret Medical has landed a European CE mark for its next-generation Sentinel Cerebral Protection System — the first system on the market that removes embolic matter following cardiac procedures, the Santa Rosa, Calif., devicemaker said Monday.
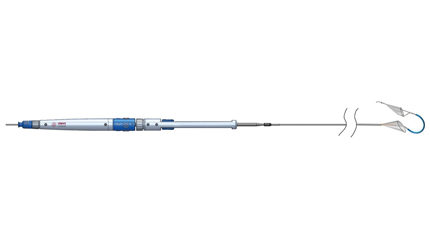
The system consists of a catheter that is inserted into the right radial artery in the right arm and threaded over the shoulder area, where it is then placed at the confluence of the brachiocephalic and right common carotid arteries. There it deploys two filters, Claret COO Tony Fields told Device Daily Bulletin.
The filters capture and remove debris launched into the bloodstream during cardiac procedures that could potentially lodge in the brain and cause strokes.
For now, Claret is focused on embolic matter dislodged during transcatheter aortic valve procedures, since such procedures have nearly three times the stroke rate of traditional surgeries, Fields said. But the company hopes to expand the system’s use to other cardiac and valve replacement procedures in the near future.
Claret is launching the product immediately in select CE mark countries, as well as in Norway and the Czech Republic, Slovakia and Poland. It is under an investigational device exemption in the U.S. and will be tested in a multicenter pivotal trial of up to 15 centers in the U.S. and Europe, the company said. — Lena Freund
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
