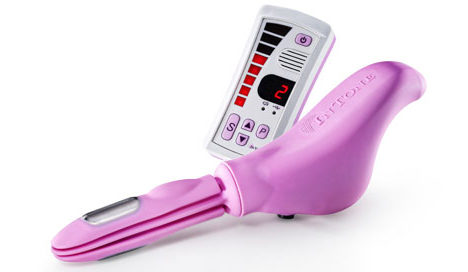
InControl Medical Gains Expanded FDA Clearance for Incontinence Device
InControl Medical said Tuesday it has received FDA clearance on an expanded indication for its InToneMV incontinence device. Originally cleared in mid-February to treat urinary incontinence in women, the device got a green light at month’s end for use in treating urinary and fecal incontinence in men and women.
The system comprises an inflatable insertion unit and a voice-guided series of pelvic floor exercises dictated through a handheld control unit. The company likens the system to in-home physical therapy, with the voice technology serving as a coach.
“InToneMV allows us to expand our product line to help both men and women suffering with urinary and fecal incontinence,” InControl CEO and founder Herschel Peddicord said.
News of the expanded indication came as InControl is launching its InTone and Apex product lines in New Zealand. In contrast to InTone and InToneMV, which treat stress, urge and mixed incontinence, Apex uses a muscle stimulation algorithm and a probe to strengthen pelvic floor muscles to prevent leakage following childbirth or menopause.
InControl plans to expand its products to other markets throughout this year, Peddicord said. — Lena Freund
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
