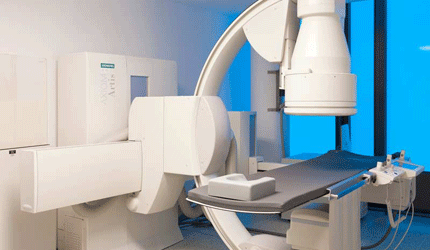
FDA Clears Shimadzu’s Trinias Angiography Systems for U.S. Distribution
Shimadzu Medical Systems USA has received 510(k) clearance from the FDA to market its Trinias family of digital angiography systems. The line includes a ceiling-mounted, flour-mounted and biplane model.
All three systems incorporate Shimadzu's ultra-high speed SCORE PRO image-processing technology, which provides real-time evaluation of noise, devices and anatomy found in both fluoro and acquisition images.
Additionally, the devices’ SCORE StentView enables real-time display of stents in a fixed position, making it easier to assess potential relationships between overlapping stents or when re-expanding a stent using a balloon, the Torrance, Calif., company says.
Trinias also incorporates seven radiation dose-reduction features that marries the need for higher image quality with the most appropriate radiation dose. The device is available in the U.S. and Caribbean. — Nick Otto
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
