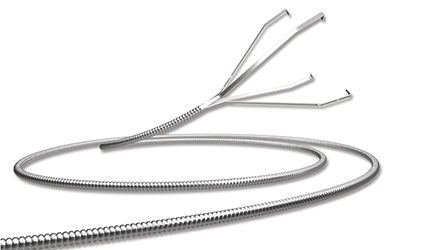
Covidien Recalls Pipeline Embolization Device and Alligator Retrieval Device
Covidien is recalling certain lots of its Pipeline embolization devices and Alligator retrieval devices due to a defect in the coating applied to the delivery wire.
According to the Irish devicemaker, the polytetrafluoroethylene coating may delaminate and detach from the devices, potentially causing embolic occlusion in the cerebral vasculature and putting the patient at risk of stroke or death.
The recall involves 32 embolization devices distributed in the U.S., Australia, France, Germany and UK, and 621 retrieval devices sold in the U.S., Australia, Canada, Europe and Latin America, the company said Friday.
The devicemaker informed customers and regulators of the defect April 1 and is arranging to replace the devices.
Covidien discovered the flaw through internal product testing. Leerink Research analyst Richard Newitter said he was told by management that the problem stemmed from a manufacturing change on the part of a supplier.
“We believe the impact is manageable and should not have an outsized impact on longer term sales/EPSS prospects, as we believe [Covidien] has identified a way to address the manufacturing issue behind the recall,” Newitter said, adding that the company has unaffected lots of both devices.
The Pipeline embolization device is used to treat adults age 22 or older with large or giant wide-necked intracranial aneurysms in the internal carotid artery from the petrous to the superior hypophyseal segments. The Alligator retrieval device is indicated for use in the peripheral and neurovasculature for foreign body retrieval. — Lena Freund
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
