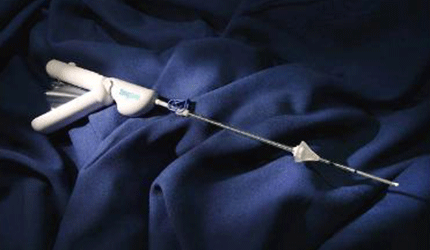
CrossBay’s SonoSure Sonohysterography and Endometrial Sampling Device Cleared in U.S., EU and Canada
CrossBay Medical said Friday that its SonoSure sonohysterography and endometrial sampling device has received marketing authorization in the U.S., EU and Canada. The device is indicated to access the uterine cavity for saline infusion sonohysterography and to obtain an endometrial biopsy, if necessary.
According to the company, SonoSure combines saline infusion hysterography and endometrial biopsy capabilities into one device, streamlining workups for abnormal uterine bleeding or heavy menstrual periods. This eliminates the need to insert a cannula into the cervix twice or schedule separate appointments for procedures that use different instrumentation, CrossBay said.
The single-use, disposable product contains a low-profile, flexible insertion catheter and a built-in endometrial sampling brush and comes with a 50-cc re-fillable fluid injection bag. Its sealing mechanism can be repositioned so as not to obscure the lower uterine segment, CrossBay noted.
SonoSure is CrossBay Medical’s flagship women’s health product. It is already available in all three regions. In the U.S., SonoSure is marketed by Norgenix. — Lena Freund
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
