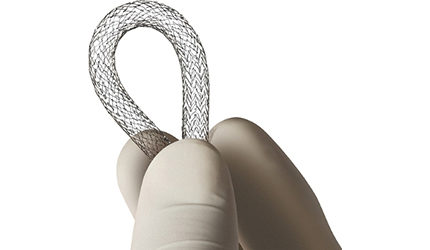
Terumo Launches Self-Expanding Stent in U.S.
Japanese devicemaker Terumo launched its Misago Rx self-expanding peripheral stent in the U.S. market last week, following receipt of premarket approval in May.
The Misago system, comprised of a nitinol stent premounted onto the distal portion of a rapid-exchange delivery catheter, is intended to treat peripheral artery disease in the superficial femoral and proximal popliteal arteries. The product has been available in territories outside the U.S since 2008.
A multicenter clinical trial — one of the first harmonization-by-doing initiatives between the U.S. and Japan — demonstrated a low potential for stent fracture, precision deployment by a single operator at the lesion site, sustained patency of almost 83 percent at one year and 88.6 percent freedom from target lesion revascularization at one year, Terumo said.
Peripheral artery disease occurs afflicts an estimated 8 million people in the U.S. — Jason Scott
