www.fdanews.com/articles/180554-masimo-gains-ce-mark-for-respiration-rate-measurement-device
Masimo Gains CE Mark for Respiration Rate Measurement Device
February 15, 2017
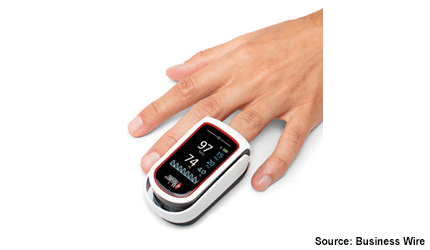
Swiss company, Masimo has received a CE mark for its MightySat Rx fingertip pulse oximeter.
The device measures and displays functional oxygen saturation; pulse rate and perfusion index with the option to add pleth variability index — allowing for automated and continuous monitoring of the respiration rate.
The device is not available in the U.S. as it does not have marketing clearance. — Cynthia Jessup

