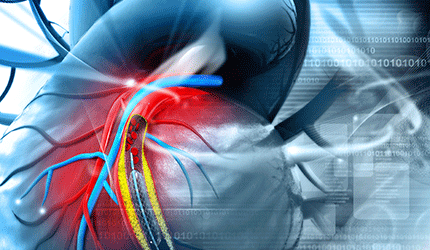
Shockwave Medical Gains CE Mark for Coronary Lithoplasty System
Shockwave Medical has achieved a CE mark for the company’s Coronary Lithoplasty system for the treatment of calcified plaque in conjunction with stenting in patients with coronary artery disease.
The device integrates angioplasty balloon catheter devices with the calcium-disrupting power of sonic pressure waves, known as lithotripsy.
Each Lithoplasty catheter uses multiple lithotripsy emitters activated after the balloon is inflated. Once activated, thee emitters produce therapeutic sonic pressure waves that are inherently tissue-selective, passing through the balloon and soft vascular tissue, preferentially disrupting the calcified plaque inside the vessel wall and creating a series of micro-fractures.
When the calcium has been broken down, the vessel can be dilated using low pressures, with minimal injury to the vessel.
The system is an investigational device in the U.S. and is not available for sale. — Cynthia Jessup

