www.fdanews.com/articles/181845-fda-clears-new-indication-for-myosciences-iovera-system
FDA Clears New Indication for Myoscience's Iovera System
May 18, 2017
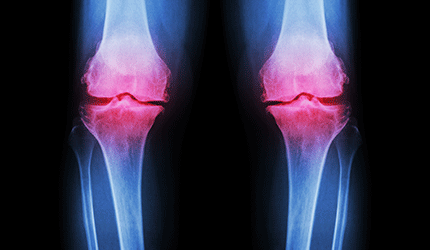
California-based Myoscience has received FDA marketing clearance for its iovera device for the relief of pain and symptoms associated with osteoarthritis of the knee for up to 90 days.
The device is a non-opioid and non-systemic treatment for blocking pain signals from peripheral nerves. The sensory nerve stops sending pain signals immediately after receiving targeted cold therapy.
The clearance was based on clinical results in which patients reported statistically significant reduction in pain and improvement in symptoms. — Cynthia Jessup

