
Home » Veniti Gets Green Light for Clinical Trials of Vein-Specific Stent
Veniti Gets Green Light for Clinical Trials of Vein-Specific Stent
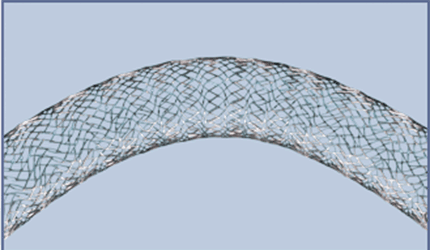
The FDA has approved an investigational device exemption for the Veniti Vici Venous Stent System, clearing the way for the St. Louis, Mo., devicemaker to begin clinical trials.
The U.S. arm of the VIRTUS clinical trial is slated to begin this fall and will enroll 200 patients at up to 30 centers, says Rodney Marcy, vice president of global sales and marketing at Veniti. The company expects to complete the trial in about two and a half years.
The European VIRTUS trial has already begun and will eventually comprise up to 86 patients, Marcy tells Device Daily Bulletin. That trial is also expected to last about two and a half years.
Hundreds of thousands of people globally suffer from lower-extremity venous disease, and the Veniti Vici System provides them with a stent specifically designed for that use, as opposed to repurposed arterial system stents commonly used in such cases, the company says.
According to William Marston, one of the principal investigators at the University of North Carolina-Chapel Hill, vein obstruction often leads to leg swelling, pain and ulceration, and vein-specific stents can reduce these symptoms while improving the cost-effectiveness of treatment.
Veniti plans to file for premarket approval following completion of the U.S. trial. — Kellen OwingsSubscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
Upcoming Events
-
23Apr
-
25Apr
-
07May
-
14May
-
30May
