
Home » ResMed Launches Air Solutions Platform of Sleep-Disorder Breathing Devices and Software
ResMed Launches Air Solutions Platform of Sleep-Disorder Breathing Devices and Software
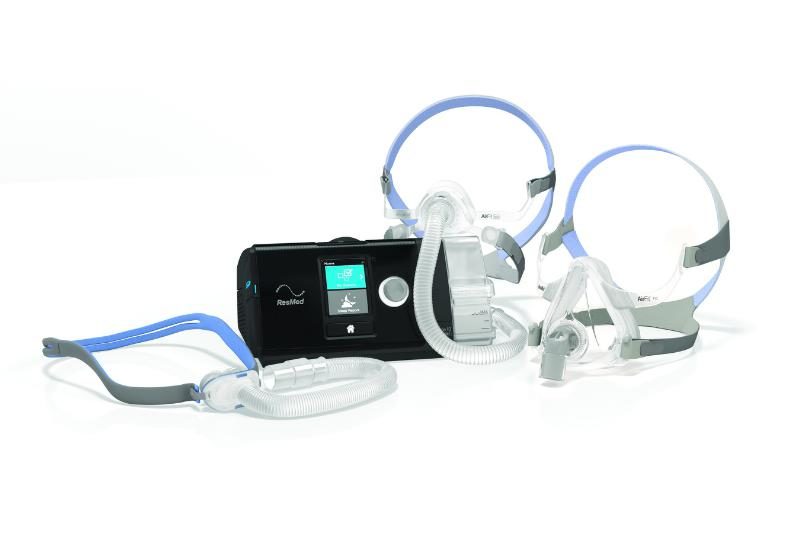
ResMed said Monday it has begun rolling out its new Air Solutions platform of sleep-disorder breathing devices, an end-to-end system of data-driven components that cover patients from diagnosis to treatment.
The platform is comprised of six components:
- ApneaLink Air: A compact and lightweight at-home sleep testing device that lets sleep labs manage each step of the diagnostic process and share data;
- AirSense10 Series: Continuous and automatic positive airway pressure devices that are compact and easy-to-use;
- AirCurve 10 Series: Devices that meet a variety of patient conditions and needs, such as extra pressure support or adaptive servoventilation;
- AirFit Masks: Easy-fitting breathing masks that include the AirFit P10 nasal pillows system, AirFit F10 full-face mask and AirFit N10 nasal mask;
- AirView Monitoring and Compliance Management System: Software that troubleshoots and detects faults in the patient management process;
- myAir Patient Engagement Application and Software: User-friendly software offering data dashboards, coaching and reinforcement messages.
The ApneaLink Air and AirFit masks are available now in the U.S., and the AirSense 10 and AirView will be available August 18, ResMed said. The AirCurve 10 Series and myAir components will be available later this year. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
Upcoming Events
-
23Apr
-
25Apr
-
07May
-
14May
-
30May
