
Home » Mevion Opens First Back-to-Back Proton Therapy Centers
Mevion Opens First Back-to-Back Proton Therapy Centers
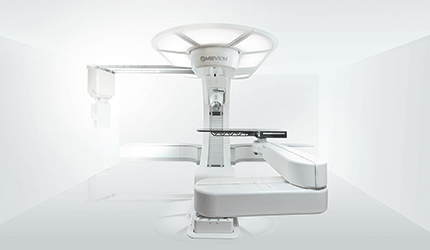
Mevion Medical Systems’ new proton therapy center has begun treating patients, less than a month after it opened its last one, making it the first company to launch back-to-back proton centers, the Littleton, Mass., devicemaker announced Monday.
Previously, proton therapy systems have cost millions of dollars to set up, usually taking up the size of a football field. This limits patient access to these facilities because few cancer centers have the money or space to build them. However, as the world’s smallest single-room proton therapy system, the Mevion S250 helps reduce these restrictions, using up to 90 percent less energy than comparable systems, the company claims.
The installation occurred at the Robert Wood Johnson University Hospital in New Brunswick, N.J. Meanwhile, the company’s proton therapy center at Ackerman Cancer Center in Jacksonville, Fla., opened April 30, is treating 24 patients per day, the devicemaker says. That facility bears the distinction of being the first ever physician-owned proton center.
The company is installing the center at four other sites in the U.S. The system is approved for use in Europe.
Proton therapy works by targeting cancer cells, but causes less damage to surrounding healthy tissue, unlike photon radiation. This makes it a more attractive option for patients with tumors in sensitive areas, such those near the heart, brain and spine. — Jason Scott
Upcoming Events
-
23Apr
-
25Apr
-
07May
-
14May
-
30May
