
Home » Centinel Grabs IDE for Cervical Disc Clinical Trial
Centinel Grabs IDE for Cervical Disc Clinical Trial
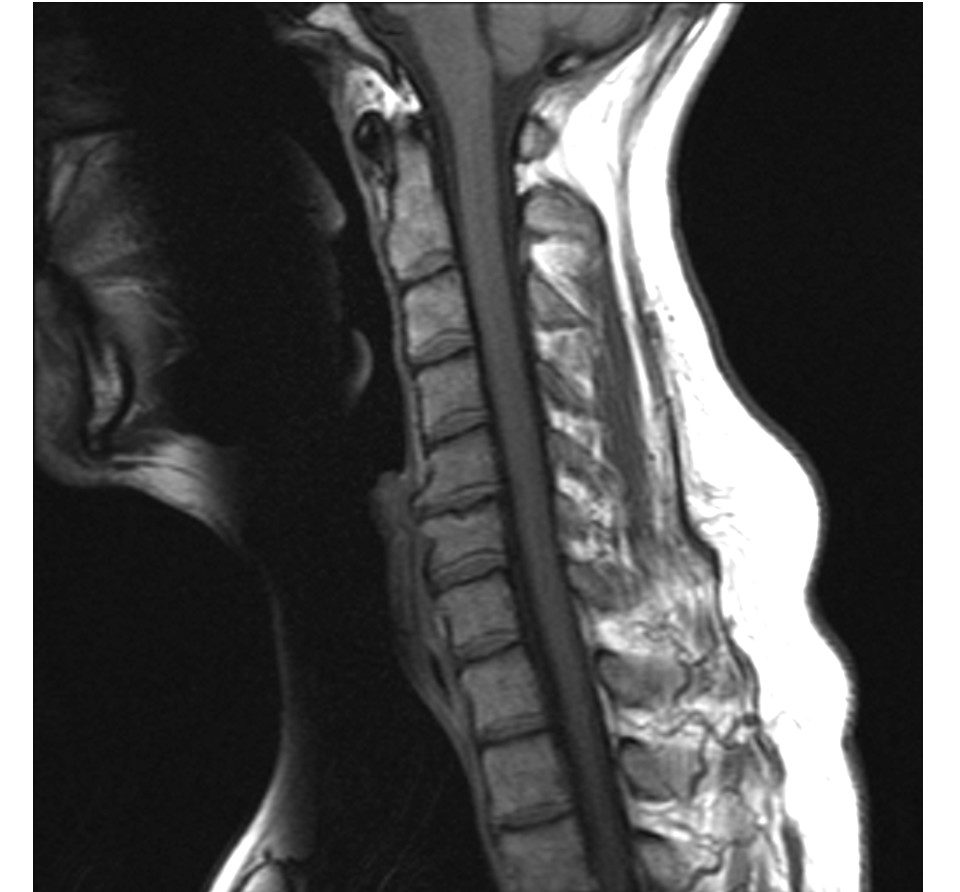
Centinel Spine has received investigational device exemption (IDE) approval from the FDA for a trial of its Prodisc cervical disc implants.
The two-level trial involves the company’s prodisc C vivo and prodisc C SK devices, two of the company’s prodisc C Anterior Cervical Total Disc products. They feature interface variations that make it easier for surgeons to match an implant to a patient’s anatomy.
The devicemaker said it plans to launch the trial immediately in multiple U.S. centers that are experienced in cervical total disc replacement (TDR) procedures.
Upcoming Events
-
25Apr
-
07May
-
14May
-
30May

