
Home » DiaSorin’s COVID-19 Test Gets European OK for Saliva Samples
DiaSorin’s COVID-19 Test Gets European OK for Saliva Samples
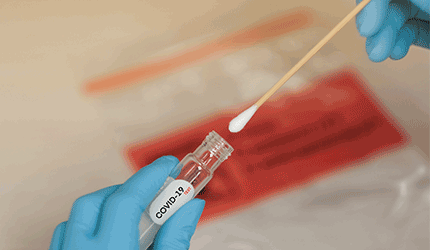
DiaSorin has earned a CE mark for its Simplexa COVID-19 Direct assay for identifying infected individuals from saliva samples.
The new clearance will give laboratories more flexibility and help them to “overcome ongoing bottlenecks, and better manage worldwide swab and transport media shortages,” the company said.
The test received an initial FDA Emergency Use Authorization in March and has since been authorized for use with nasal swabs, nasopharyngeal swabs, nasal wash/aspirates and bronchoalveolar lavages, the company said.
Upcoming Events
-
07May
-
14May
-
30May

