Boston Scientific Rolls Out OffRoad Re-Entry Catheter System in the U.S.
Boston Scientific Wednesday announced the U.S. launch and first use of its OffRoad Re-Entry Catheter System. The system helps physicians perform stenting or angioplasty in patients with advanced peripheral artery disease by allowing them to travel through the vessel wall to get around chronic total occlusions.
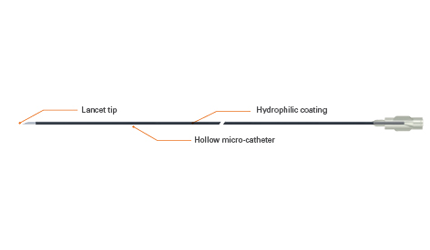
The OffRoad system consists of a guidewire that slides around the blockage by dissecting the intimal layer of the vessel and moving into the subintimal channel. A positioning balloon catheter then moves over the guidewire into the subintimal channel using a radiopaque marker, providing the surgeon with a visual image that aids in accurate placement, the company said.
The surgeon then removes the guidewire and threads a microcatheter lancet through the positioning balloon, which inflates and rotates toward the true lumen. A .36 mm guidewire advances through the lancet, achieving true lumen re-entry and allowing the procedure to go ahead, Boston Scientific said.
J.A. Mustapha, director of cardiovascular research at Metro Health Hospital in Wyoming, Mich., and the first surgeon to use the device following the launch, said, “The unique design of the OffRoad System facilitates re-entry, giving me confidence that I will be able to successfully deploy the tools I need to treat the blockage.”
OffRoad was cleared by the FDA in late 2013 based on the Re-ROUTE trial, in which 84.8 percent of patients were successfully treated. — Lena Freund
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
