MedMira Gets Go-Ahead to Market Reveal HIV Test in Mexico
Halifax, Nova Scotia, devicemaker MedMira is launching its Reveal HIV test in Mexico via distribution partner Diagno Medical, following approval by Mexico’s regulatory authority, COFEPRIS.
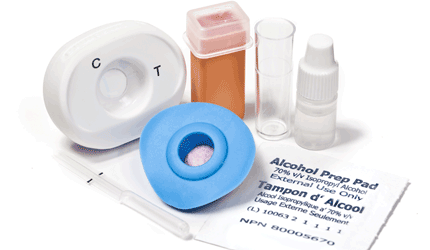
According to MedMira, the Reveal HIV kit eliminates the need for physicians to manage inventories of testing kit parts, creating greater operational and cost efficiencies. The 1.28 ounce kit includes all the necessary ingredients to test patients anytime and anywhere: lancets, alcohol swabs and a test cartridge built not to leak, the company said. It is stored at room temperature.
The test uses the company’s Rapid Vertical Flow technology, producing results in less than two minutes, MedMira said. A blood or serum sample is taken, using an absorbent pad, and when antigen and antibody meet, the reaction leaves a visual mark on the membrane.
The test has a 99.9 percent sensitivity rate and a 99.8 percent specificity rate, MedMira spokeswoman Andrea Young told Device Daily Bulletin.
Reveal HIV will be sold across Mexico to hospitals, laboratories, major urban clinics and mobile clinics in rural locales, Young said. Approximately 220,000 people are living with HIV in Mexico, more than 30 percent of whom live in areas where HIV testing is not easily accessible, according to the AIDS Healthcare Foundation.
The kit is available in the U.S., Canada, Africa, the Middle East and the EU, and MedMira is seeking regulatory clearance in Brazil, Ecuador, Panama and Argentina as well. — Lena Freund
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
