FDA Approves Abbott Stent for Blocked Leg Vessels
The FDA has granted premarket approval for Abbott’s Supera stent to treat people with blocked blood vessels in the leg — securing its place in the one of the fastest-growing segments of the peripheral stent market, the company says.
The device improves luminal diameter in patients with symptomatic de novo or restenotic native lesions or occlusions of the superficial femoral artery and/or proximal popliteal artery with reference vessel diameters of 4.0 mm to 6.5 mm and lesions lengths up to 140 mm, according to the Illinois devicemaker.
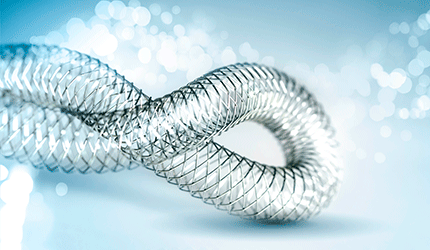
Rather than resisting the artery’s natural movements, Supera mimics them, which helps to reduce pain in the legs while walking, Abbott says. The stent is made from six closed-end, interwoven nitinol wires mounted on a 6 Fr or 7 Fr delivery system. It is compatible with 0.014” and 0.018” guidewires and comes in 80 cm and 120 cm lengths.
Abbott claims Supera is more flexible than other nitinol stents and more efficiently resists kinking or fracturing under pressure.
The stent was tested in an interventional Phase III study of adult patients at 49 centers across the U.S. Clinical results showed no stent fractures within the first year and only 0.5 percent after two years. The study was sponsored by IDEV Technologies, which Abbott acquired last year.
Supera also is CE-marked for sale in Europe. — Lena Freund
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
