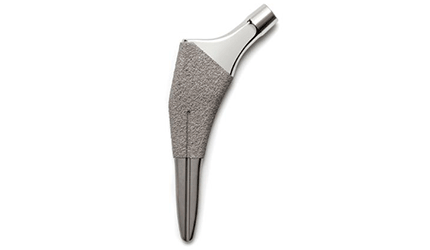
FDA Clears Ortho Development’s Ovation Tribute Hip Stem
Ortho Development, a Draper, Utah, devicemaker, said Wednesday that the FDA has cleared its Ovation Tribute Hip Stem femoral hip prosthesis for cementless use in total hip replacement and hemiarthroplasty procedures.
The device is designed as a line extension of the current Ovation hip stem and meant for use with the company’s femoral heads and compatible acetabular components. Ovation Tribute’s instrumentation platform is broach-only, the company said.
New features allow surgeons to take multiple approaches, including the direct anterior approach, when implanting the prosthesis, the company added. The prosthesis is a tapered wedge with a short stem available in 12 sizes with both standard and extended neck offsets to better accommodate patients’ individual anatomies.
Ovation Tribute is currently being marketed by a network of distributors in the U.S. It is also available in Japan. — Lena Freund
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
