FDA Clears Jintronix’ Motion Capture Rehabilitation System
Seattle-based Jintronix plans to launch its Microsoft Kinect-based rehabilitation system in the third quarter of 2013, following receipt of FDA 510(k) clearance.
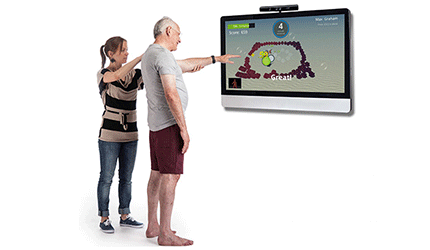
The Jintronix system combines evidence-based treatments, virtual games and motion tracking sensors to enhance physical therapy. Patients receive instant feedback on their success or failure at performing an exercise, and therapists are able to able to track and update exercises as progress is made. The aim is to increase the patient’s motivation to stay on track by making the program fun and interactive, the company said.
"As consumers become more familiar with technologies such as motion capture and facial recognition, we think the opportunities for consumer-based technologies to change healthcare are enormous,” Jintronix said. “Our solution for the Kinect is an important entrant.”
While full prices have not been set, the sensor retails for $249.99, said Daniel Schacter, the company’s co-founder.
This is the first time the Kinect software has been approved for use directly by patients in a healthcare setting. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
