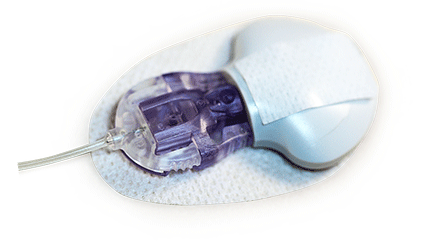
Medtronic Launches Combo Insulin Pump, Glucose Monitor in Europe
Medtronic Tuesday announced the CE marking and European launch of its MiniMed Duo, a two-in-one on-body device that combines an insulin pump with a continuous glucose monitor.
Insulin pumps provide precise, 24-hour insulin delivery, and pumps integrated with a CGM are clinically proven to provide better glucose control than multiple daily injections or pump therapy alone, the Minneapolis devicemaker says.
According to Medtronic, MiniMed Duo provides diabetics with real-time glucose values and customizable alerts that prompt the wearer to take action before glucose levels go too high or too low. Better glucose control reduces the risk of complications such as eye and kidney disease and nerve damage.
MiniMed Duo is easy to use. The glucose sensor and insulin-delivering cannula are both inserted via a single insertion device, and wearers only have to remember to change one device every three days.
The system takes up less space on the body than conventional pumps and monitors, accommodates movement better, and provides a single-button insertion and automatic needle retraction, so that patients don’t have to see the needle, Medtronic says. Ninety-six percent of people using the device rated insertion pain as 0 or 1 out of 10.
MiniMed Duo will launch first in the UK and be made available in select European countries over the next few months, depending on local approvals, Medtronic spokeswoman Karrie Hawbaker tells Device Daily Bulletin.
Hawbaker says the company has not established a timeline for bringing MiniMed Duo to the U.S. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
