Itamar Medical Gains FDA Clearance for Sleep Apnea Device
Itamar Medical said Monday the FDA has cleared the upgraded version of its WatchPAT sleep apnea device, WatchPAT Unified.
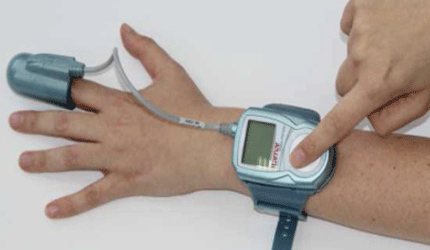
The sleep diagnostic allows the test allows a person’s oxygen levels and peripheral arterial tone (PAT) to be measured using a single finger and a unified probe. This simplifies the test procedure while improving reliability and user comfort, the Israeli devicemaker said.
Earlier WatchPAT models had two finger sensors, one for measuring PAT and the other for monitoring blood oxygen saturation.
The device is used at home to diagnose sleep breathing disorders. The PAT signal is measured noninvasively through the finger tips using a custom programmed sensor and signal processing algorithms, the company said. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
