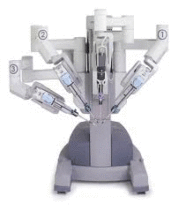
Intuitive Surgical’s da Vinci Xi Surgical Platform Wins CE Mark
Intuitive Surgical on Wednesday announced the receipt of CE Mark approval for its da Vinci Xi system, a surgical platform that allows for complex surgery to be performed in a minimally invasive way.
The system received 510(k) clearance from the FDA in April and is available throughout the U.S. With the CE Mark, the device can be marketed in all countries that observe that designation.
According to the Sunnyvale, Calif., devicemaker, da Vinci Xi accommodates and integrates technologies such as fluorescence imaging, advanced instruments and anatomical access. It also is designed to accommodate future innovations.
The system will replace large-incision open surgeries in the areas of the pelvis, abdomen or chest with a minimally invasive approach, Intuitive says, adding that da Vinci Xi aims to reduce the costs and complications associated with open procedures.
The company is seeking additional regulatory approvals to market the system worldwide. The FDA cleared Intuitive’s EndoWrist One vessel sealer instrument, designed for use with da Vinci Xi, earlier this month. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
