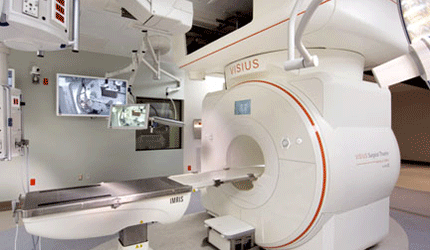
IMRIS’ Visius Surgical Theatre Wins CE Mark
Minneapolis devicemaker IMRIS said Wednesday that it received a CE mark to integrate its next-generation magnetic resonance imaging technology into the Visius Surgical Theatre. The new MRIs provide better image quality, faster 3D image acquisition and improved ease of use during intraoperative MRI procedures, the company says.
The imaging technology is based on the Siemens Aera 1.5T (tesla) and the Skyra 3.0T MRI scanners, and was cleared by the FDA in February. With the CE mark, the device can be sold and marketed in all countries that observe that designation.
According IMRIS, the Visius surgical theatre plus high-field iMRI gives surgeons on-demand, real-time data and state-of-the-art imaging throughout a procedure as the scanner slides along ceiling-mounted rails.
The system will be used predominately in neurosurgical centers for brain tumor resections, laser ablation for tumors and epilepsy, deep brain stimulation and other minimally-invasive techniques to improve patient outcome, said IMRIS President and CEO Jay D. Miller.
The company also makes intraoperative CT scanners, head-fixation devices, imaging coils and operating room tables for use with the Visius system. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
