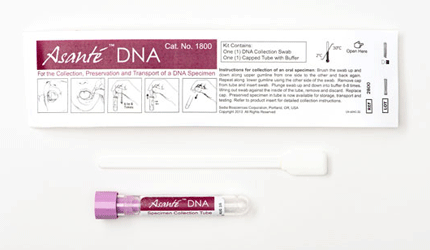
Sedia Biosciences Launches Cost-Competitive DNA Collection, Purification Kits
In vitro diagnostic and epidemiological test maker Sedia Biosciences has launched its Asante DNA Specimen Collection Kit and Asante DNA Purification Kit in the U.S. The kits are a cost-competitive alternative to current methods of DNA collection, the company says.
The collection kit features low DNA retention, few interfering substances and an easy-to-use design, according to Sedia. The collected samples are suitable for testing by most in vitro DNA amplification methods, including polymerase chain reaction and modifications, pretreatments and extensions, helicase-dependent amplification, ligase chain reaction and loop-mediated isothermal amplification, the company says.
The purification kit helps to simplify extraction, purification and concentration of DNA collected with the specimen kit by reducing the need for abrasive materials, chemical reagents or extreme concentration methods used by other DNA collector manufacturers, the Portland, Ore., company notes.
These features enable the company to produce the device at a lower cost and pass the savings on to its customers, says Ronald Mink, president and chief science officer.
As a Class I device, no FDA approval was required, Mink notes, adding the kits are registered with the FDA. Rollout, which has already begun, will be ramped up in the coming weeks, he says. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
