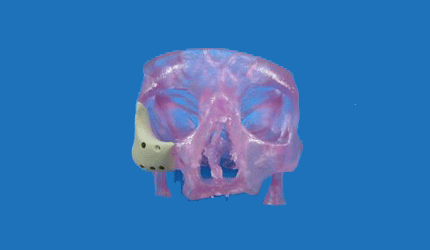
Oxford Performance Materials' 3D-Printed Facial Implant Clears 510(k) Hurdle
Advanced materials and 3D printing company Oxford Performance Materials will launch its 3D-printed OsteoFab Patient-Specific Facial Device in the U.S. immediately, following receipt of FDA 510(k) clearance. The device is the first and only polymeric implant for facial implants cleared for sale in the U.S., the South Windsor, Conn., company said Tuesday.
Biomet will service as the devices’ global distributor.
The OPSFD implants are biocompatible in that they are mechanically similar to bone, radiolucent and support bone attachment, the company says. They will be 3D-printed using the company's OsteoFab process, which combines laser sintering additive manufacturing technology with OPM’s OXPEKK powder formulation to print orthopedic and neurological implants.
OPM expects the device to reduce surgical times, hospital stays and procedure complications.
CEO Scott DeFelice called the clearance groundbreaking. “We now have the ability to treat these extremely complex cases in a highly effective and economical way, printing patient-specific maxillofacial implants from individualized MRI or CT digital image files from the surgeon, “ he said.
Clearance of the OPSFD follows the February 2013 clearance of OPM’s OsteoFab Patient-Specific Cranial Device. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
