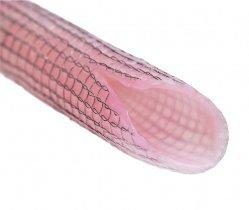
Kips Bay Medical Gets Updated CE Mark for Mesh Technique
Minneapolis, Minn., devicemaker Kips Bay Medical said Monday it has received an updated CE mark for a new surgical implant technique for the eSVS Mesh in coronary artery bypass grafting.
Instead of incorporating the mesh inside saphenous vein grafts, it will be fitted like a sleeve over the grafts to strengthen them during coronary artery bypass graft surgery, Scott Kellen, chief financial officer at Kips Bay, tells Device Daily Bulletin.
The changes are intended to reduce the variables in the eMESH I clinical feasibility trial and the risk of early graft occlusion, the company explains. It will also simplify the process of applying and implanting the eSVS Mesh, reducing procedure costs, Kips Bay says.
The eMESH I trial, which is underway in the U.S. and five European countries, aims to demonstrate the initial safety and performance of the eSVS Mesh as an external saphenous vein graft support device during CABG surgery. The company hopes to use the results to support an investigational device exemption request with the FDA. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
