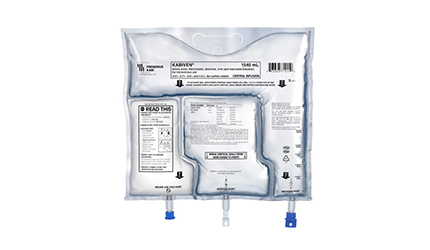
Fresenius Kabi's Parenteral Nutrition Three-Chamber Bags Get FDA Nod
The FDA has cleared Fresenius Kabi’s Kabiven and Perikabiven parenteral nutrition products in a three-chamber bag, the only such container of its kind available in the U.S., the I.V. and infusion products maker said Tuesday.
The products are intravenously infused solutions of amino acids, electrolytes, lipids and dextrose delivered in a bag that allows portions to be measured out in appropriate volumes and concentrations, the German company says.
The three-chamber bag keeps the macronutrients and micronutrients stable until the bag is activated and the contents are mixed for patient use, the company adds.
The products will be especially useful for home-based patients. “For people who require long-term parenteral nutrition at home, Kabiven and Perikabiven are particularly beneficial because they provide needed nutrition, including lipids, and don’t require refrigeration, making travel more convenient,” says Darlene Kelly, advisor at the Oley Foundation for Home Parenteral and Enteral Nutrition. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
