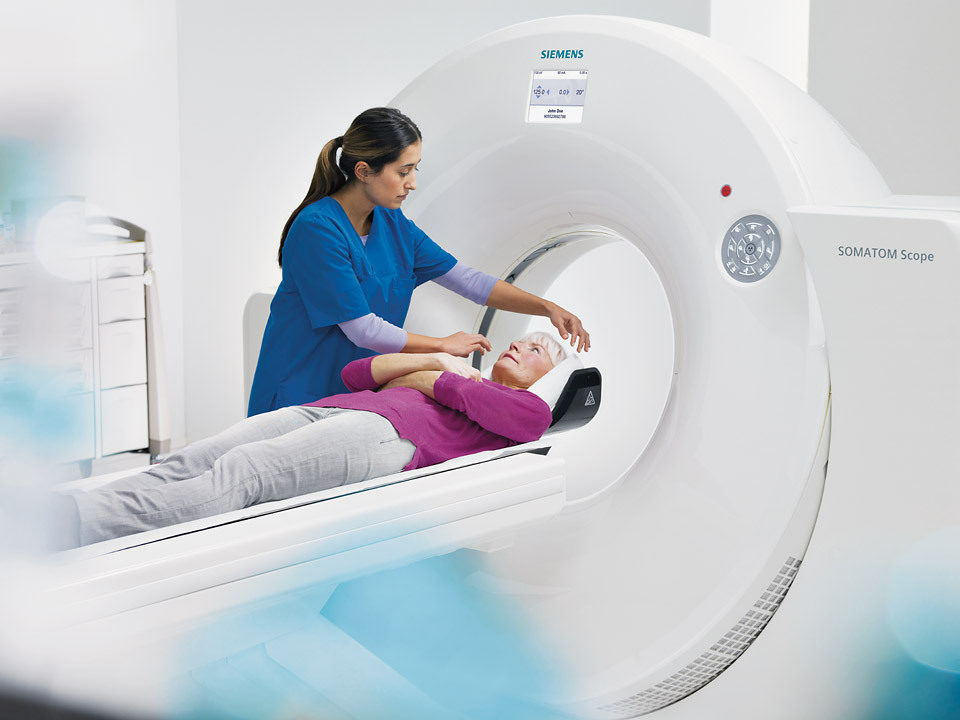
Siemens Somatom Scope CT Scanner Gets FDA Green Light
Siemens Healthcare has received FDA clearance for its Somatom Scope — a 16-slice computed tomography (CT) scanner. The scanner is designed for use in routine diagnostic imaging, oncological examinations, interventional radiology and vascular imaging, the company said last week.
The Somatom Scope delivers high image quality and dose reduction at a price point that allows for more patients to receive diagnostic imaging services, Siemens said. It is available in two configurations that allow healthcare providers to choose the proper dosing for patients.
The system also includes technology that extends the scanner’s operational lifetime by minimizing wear and tear on its most vital components, Siemens said.
Murat Gungor, vice president of CT and radiation oncology at Siemens, said the system will give more patients access to the company’s technologies, while allowing facilities to control diagnostics costs. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
