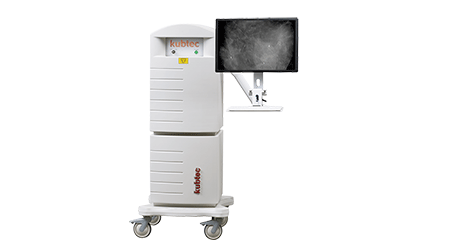
Kubtec Nabs FDA Approval of Breast Specimen Radiography System
Milford, Conn., devicemaker Kubtec said Tuesday that its MOZART breast specimen radiography system is now available worldwide, after it won FDA approval.
The MOZART system is the first to use tomosynthesis technology, which allows 3D assessment in one step without having to turn and reposition the specimen container. This lets the system to capture an entire data set in less time than it takes to acquire multiple 2D images, according to Kubtec President Vikram Butani.
Kubtec maintains the system provides more complete analysis of removed breast tissue than other available systems, helping to reduce rates of re-excision and patient callback rates. The system also takes less time to provide high-resolution images, which results in shorter surgeries and less anesthesia time for patients, reducing the risk of infection, the company says.
The mobile system can be located in radiology, pathology or surgery units, and images can be viewed within seconds via wireless transmission or directly on the system’s monitor, the company says. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
