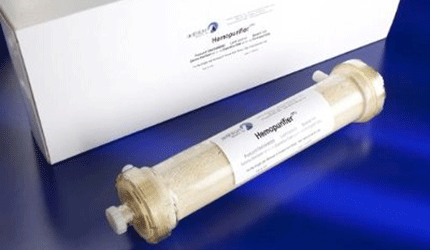
Aethlon Medical’s Hemopurifier Used to Treat Ebola Patient
Doctors in Germany used Aethlon Medical’s biofiltration technology to treat a patient infected with the Ebola virus, following emergency use authorization from the German government.
The Hemopurifier targets the rapid elimination of viruses and immunosuppressive proteins by filtering infected blood through a bundle of hollow, porous fibers enclosed in a cylindrical plastic case, explains Aethlon President Rod Kenley. Pressure from a blood pump pushes a fraction of the plasma through a porous membrane, leaving the cellular components of the blood inside the fibers.
Once this is done, the virus passes through the membrane’s pores and is absorbed by affinity resin just outside the fibers. The plasma then reenters the fibers and recombines with the blood, which is reintroduced into the patient with a much lower concentration of viruses.
Kenley said the device was based on an off-the-shelf plasma filter, and is similar to an artificial kidney.
The treatment reduces the viral load more rapidly than drug therapy, enhancing the immune system’s ability to attack and overcome the diminished amount of the virus, he adds.
Originally developed to treat HIV and hepatitis C, the Hemopurifier has been the subject of clinical trials in India since 2007. In a recent trial there, it cured five out of seven genotype 1 HCV patients, according to Kenley.
The FDA granted Aethlon approval to evaluate the Hemopurifier as a treatment for HIV and HCV in 2013. More recently, the agency authorized the device’s use as an emergency intervention for Ebola patients.
Studies of the device in Ebola-infected monkey blood and cell cultures, conducted at the United States Army Medical Research Institute for Infectious Diseases, showed it to be effective in binding and extracting the virus from blood. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
