FDA Green Lights St. Jude’s TactiCath Quartz Ablation Catheter for AFib
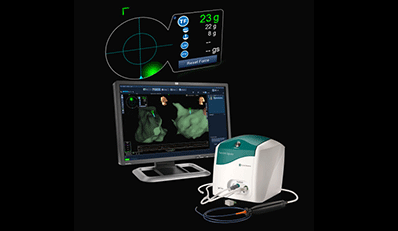
St. Jude Medical Monday announced FDA approval of its TactiCath Quartz irrigated ablation catheter with contact-force technology, which lets physicians change the force the catheter applies to a patient’s heart wall during an ablation procedure.
The thin, flexible catheter allows doctors to monitor the pressure the catheter tip exerts on the heart wall. This helps in creating more effective lesions for patients with paroxysmal atrial fibrillation, St. Jude said.
In clinical trials, 85.5 percent of patients who received an ablation with TactiCath were free from paroxysmal AF after 12 months, compared with 67.7 percent of patients in which contact-force parameters weren’t used, the St. Paul, Minn., devicemaker said.
Without contact-force technology, physicians estimate by touch the amount of force that is being applied during an ablation. If too little force is applied, an effective lesion may not be created, causing AF to possibly recur. If too much force is used, potentially serious tissue injury may occur, St. Jude said.
According to the company, about 2.7 million people in the U.S. experience AF, making it the most common type of arrhythmia. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
