Kubtec’s Neonatal Low-Dose X-Ray System Gets FDA Green Light
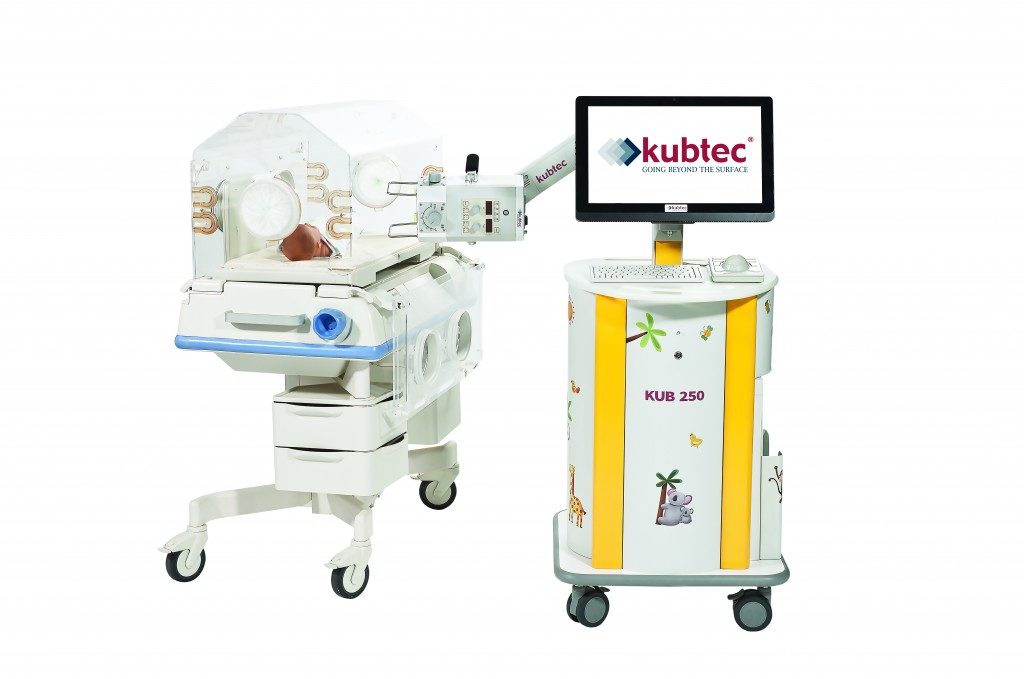
Kubtec, a maker of low-dose digital radiography systems, said Friday that it received FDA approval for its low-dose portable digital X-ray system. The KUB 250, which was developed for neonatal intensive care units, will launch at the beginning of December.
The high resolution, compact and lightweight imaging system helps to track subtle changes in pathology while maintaining a low dose of radiation, which is important when imaging high-risk infants in the NICU, Kubtec says. According to Chester Lowe, director of R&D, the KUB 250 has been clinically tested to reduce radiation exposure by up to 40 percent.
The system also features an articulating arm and a lightweight digital radiography detector that slides into the incubator slot, eliminating the need to move the newborn.
According to the Milford, Conn., devicemaker, existing X-ray systems sacrifice high resolution in order to keep the radiation dose low, resulting in poor image quality and the need for additional X-rays, which defeats the aim of low-dose X-rays. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
