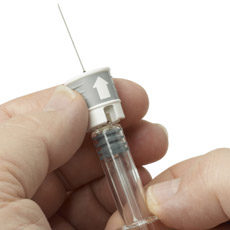
Raumedic-Cambridge Design Partnership’s Needle Safety Device Wins Coveted Award
Medical device component maker Raumedic AG and Cambridge Design Partnership received the 2014 DeviceMed award for most innovative device for their RauSafe needle safety device. The device features a telescopic sleeve that is activated after injection, covering the needle and protecting caregivers from needlestick injuries.
Approximately 385,000 needlestick injuries occur annually in U.S. hospitals, according to the companies. However, many prefilled glass syringes still don’t meet federal safety regulations aimed at reducing them and manufacturers face lengthy and costly processes to redesign and revalidate needle-safe primary pack delivery devices.
Helmbrechts, Germany-based Raumedic saw the need for a needle safety device that could be retrofitted to existing primary packs without revalidation, and partnered with CDP to develop it.
RauSafe is currently available for license.
The award was announced at Compamed 2014, the international trade fair for the medical engineering supply industry. — Kellen Owings
Subscribe to Devices & Diagnostics Letter for complete coverage of the medical devices industry. Click here for more information.
