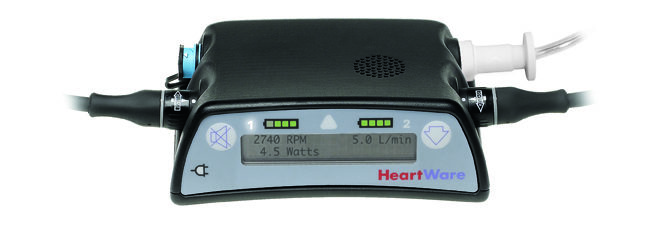
HeartWare Issues Voluntary Recall of Ventricular Assist System Controllers
HeartWare International issued a voluntary urgent recall in the U.S. of older versions of its Ventricular Assist System Controllers, due to their propensity for electrostatic discharge. Such an event could cause a heart pump to stop, resulting in serious injury or death, the company said Friday.
The recall affects controllers with serial numbers CON000001 through CON005472. These devices were distributed during HeartWare’s Advance and Endurance clinical trial prior to their 2012 approval. The company estimates that about 120 patients are affected by the recall. Newer controllers have been enhanced to minimize the problem and, therefore, aren’t being recalled.
Physicians should identify patients with these controllers, review the risks with them and exchange the controllers for ones with serial numbers CON005473 or higher, if medically advisable, HeartWare said.
The company said it has alerted the FDA to the recall. — Kellen Owings
