Somna Therapeutics Gets FDA Green Light for Silent Reflux Treatment
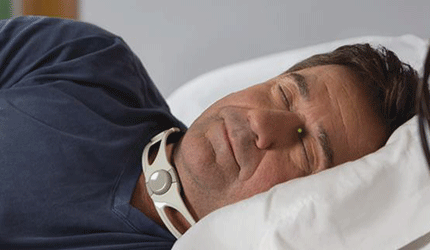
The FDA granted 510(k) clearance to Somna Therapeutics’ Reza Band, a noninvasive device for the relief of discomfort from silent reflux. Also known as laryngopharyngeal reflux, the condition causes heartburn, hoarseness, acid reflux, persistent cough and irregular breathing.
Meant for nighttime wear, the Reza Band prevents regurgitation within the upper esophageal sphincter by administering light pressure to the cricoid cartilage, according to the Germantown, Wis., company.
In a multicenter clinical trial, 86 percent of users saw significant reduction of their symptoms after two weeks, the FDA said. Moreover, 92 percent of doctors and 75 percent of patient expressed satisfaction with the device.
If untreated, silent reflux may lead to chronic ear infections and fluid build-up in the middle and inner ear, among other issues. In severe cases, surgery is necessary. However, the Reza Band will potentially cut down on such cases while providing noninvasive relief.
Reza Band, Somna’s first FDA-cleared product, is available nationwide via a doctor’s prescription. The company was founded in 2012 in partnership with the Medical College of Wisconsin. — Jason Scott
