FDA Approves Medtronic’s CoreValve System for Transcatheter Valve-in-Valve Procedures
Medtronic has won an expanded FDA indication for its CoreValve System for valve-in-valve procedures in which patients’ surgical aortic heart valves no longer function, the Dublin, Ireland, devicemaker announced Tuesday. The system ranks as the first U.S.-approved transcatheter heart valve for VIV procedures in high- and extreme-risk patients.
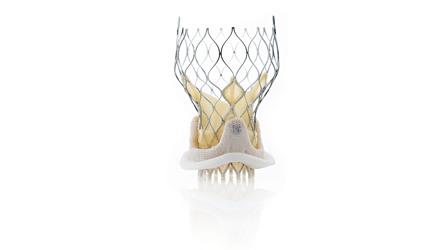
The CoreValve System — featuring a supra-annular valve design that helps maximize blood flow in patients whose artificial heart valves demonstrate stenosis and/or regurgitation — is implanted inside a failing surgical heart valve, having an inner diameter from 17-29 mm through a low-profile, 18 Fr delivery catheter, approved for use with each CoreValve size (23 mm, 26 mm, 29 mm and 31 mm) and three methods of delivery (transfemoral, subclavian and direct aortic).
An estimated 200,000 people receive surgical aortic valves annually, with the valves having a lifespan of 15 years or more. However, as surgical valves break down over time, a patient might need another valve replacement. This poses a problem for those too sick or weak to undergo open-heart surgery again, making the transcatheter VIV procedure an ideal option for these patients, the company said.
A multicenter clinical study demonstrated low rates of mortality and stroke — combined rates of 4.2 percent at 30 days and 10.7 percent at six months — with significantly improved hemodynamics and quality of life for failed surgical heart valve patients. Additionally, the largest global VIV registry also demonstrated significantly improved hemodynamics, with a decreased blood flow resistance level. The latter study boasted a one year follow-up survival rate of 89 percent, similar to other non-VIV transcatheter aortic valve replacement studies, the devicemaker said.
In 2014, the FDA approved the CoreValve System in patients at high- and extreme-risk for surgery. The system gained the CE Mark in 2007, being given an expanded indication for VIV procedures in 2013. CoreValve also won Japanese regulatory approval for patients with severe aortic stenosis unable to have surgery.
The CoreValve System has been implanted in more than 75,000 people in more than 65 countries. — Jason Scott
