Cardiovascular Systems’ Smaller Diamondback 360 Wins FDA Nod
Cardiovascular Systems has won FDA 510(k) clearance for a smaller version of its Diamondback 360 system for peripheral artery disease treatment, the St. Paul, Minn., devicemaker announced Monday.
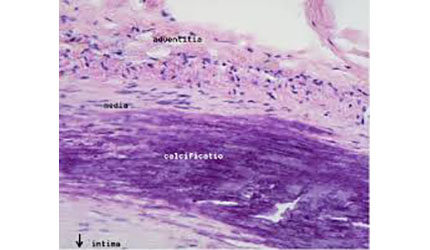
The system comes in either 1.25-mm solid or micro crown versions, which attach to a shaft and sand away plaque, including calcified lesions, while leaving healthy tissue intact. The device features a more flexible shaft than its previous iteration.
The system’s 4-French sheath compatibility means health professionals can use it to treat PAD with smaller incisions at below the knee sites.
The Diamondback system was FDA-cleared for peripheral artery treatment in 2007. It received an expanded indication for coronary arteries in 2013. The company’s Stealth 360 atherectomy system for severe PAD was CE Mark-approved in November.
PAD occurs when lower extremity arteries contract or contain blockages and can cause severe pain, decreased mobility and non-healing leg ulcers. Serious health consequences may result, including amputation, cardiovascular disease and death. — Jason Scott
