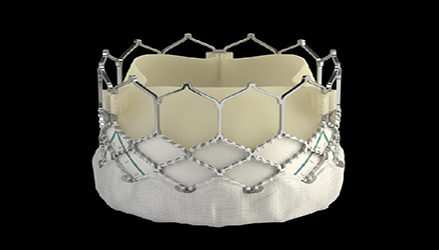
Sapien 3 Artificial Heart Valve Nabs FDA Nod
Edward Lifesciences’ Sapien 3 transcatheter heart valve for patients with aortic valve stenosis won FDA 510(k) clearance Wednesday.
The valve is indicated for patients who can’t be operated on or are high risk for death or complications with open-heart surgery. It is contraindicated for patients who can’t tolerate anticoagulation or antiplatelet therapy.
This is the Irvine, Calif., devicemaker’s third generation of the product, which was FDA-approved in 2011. The new version features a skirt at the base of the valve meant to minimize leakage around the device.
In multicenter clinical trials, the valve displayed a reasonable assurance of safety and effectiveness, the FDA says. Still, patients can face risk of serious complications.
The company says FDA approval came earlier than expected, causing it to ramp up supply significantly. The launch should be completed by the end of this year.
The Sapien 3 THV has been available in European markets since 2014. — Jason Scott
