www.fdanews.com/articles/180658-fda-grants-approval-to-intersect-ents-steroid-releasing-implant-device
FDA Grants Approval to Intersect ENT’s Steroid Releasing Implant Device
February 24, 2017
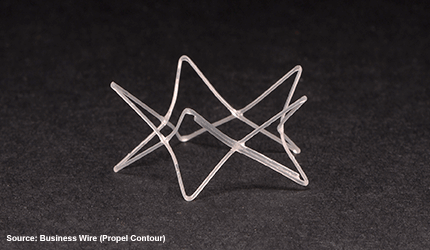
California–based, Intersect ENT has received FDA approval for Propel Contour, a dissolvable, steroid- releasing implant device that facilitates treatment of patients with chronic sinusitis in the frontal and maxillary sinuses.
The device is designed to conform to the sinus ostia, has a flexible delivery system to make it easier to access tight areas of the sinus anatomy and delivery the steroid where it is needed in order to maximize sinus surgery outcomes.
The company expects to launch Propel Contour in the second quarter of 2017. — Cynthia Jessup

