www.fdanews.com/articles/181131-fda-awards-marketing-clearance-to-bodycads-personalized-unicompartmental-knee-system
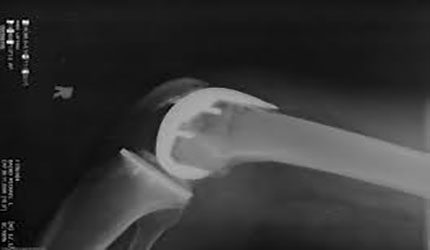
FDA Awards Marketing Clearance to Bodycad’s Personalized Unicompartmental Knee System
March 30, 2017
Quebec–based Bodycad (UKA) has received FDA marketing clearance for its Bodycad unicompartmental knee system, a joint reconstruction implant system.
The device is designed to the patient's anatomical features and kinematics. The system is based on proprietary 3D rendering of medical images of the patient's anatomy.
Recent Bodycad studies of the UKA have demonstrated positive outcomes, shorter hospital stays versus total knee arthroplasty and lower 30-day readmissions. — Cynthia Jessup

