www.fdanews.com/articles/196095-dxterity-earns-ce-mark-for-finger-stick-blood-collection-tube
DxTerity Earns CE Mark for Finger Stick Blood Collection Tube
March 3, 2020
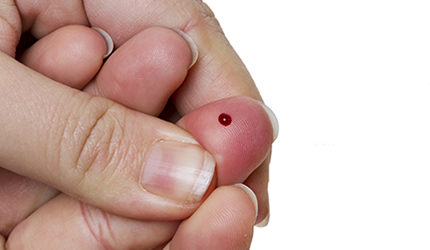
DxTerity received CE Mark certification for its DxCollect MicroCollection tube, a device used to collect and store finger stick blood samples that are used in genomic testing.
The tube is capable of transporting up to 100 microliters of blood taken with a finger prick for DNA and RNA-based genomic testing.
The device can stabilize samples at room temperature and can be shipped in ambient conditions using standard mail, the company said.

